Molecules on surfaces
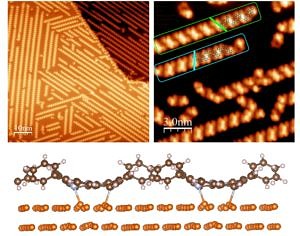
To unravel the adsorption site of a molecule on a surface is one of the most prominent tasks when studying adsorbate-surface interaction. For small molecules or atoms this is relatively straight forward since there are not many inequivalent configurations to consider. For larger molecules like the porphyrin molecule shown here it is more appropriate to speak of an adsorption geometry since the molecule can bind to the surface by more than one atom-atom bond and internal degrees of freedom (twisting, distortions) come into play.
Attractive molecule-molecule interactions are the cause of self-assembly patterns like the spontaneous chain formation of the 2H-DPP molecules on Cu(111) when deposited at room temperature. The identification of such structures requires STM investigations with sub-molecular resolution. To understand and steer such pattern formation requires the analysis of the hierarchy of interaction energies, e.g. the relation between diffusion barriers caused by molecule-substrate interactions and molecule-molecule interaction. The DFT calculation of such energies become quite involved and the experiments are important benchmarks of the quality of the inevitable approximations employed in DFT.
A special focus of our work is the adsorption of larger organic molecules on thin oxide films on metal substrates. Here, the challenge for experiment and theory is even greater. The efforts in this direction are part of the research unit “funCOS”.
Contact
- Prof. Dr. M. Alexander Schneider (Website: https://www.fkp.physik.nat.fau.eu/person/m-alexander-schneider/)
Publications
- Xiang, Feifei, Anja Gemeinhardt, and M. Alexander Schneider. "Competition between Dehydrogenative Organometallic Bonding and Covalent Coupling of an Unfunctionalized Porphyrin on Cu(111)." Acs Nano 12.2 (2018): 1203-1210.
- Lepper, Michael, et al. "Adsorption Behavior of a Cyano-Functionalized Porphyrin on Cu(111) and Ag(111): From Molecular Wires to Ordered Supramolecular Two-Dimensional Aggregates." Journal of Physical Chemistry C 121.47 (2017): 26361-26371.
- Lepper, Michael, et al. ""Inverted'' porphyrins: a distorted adsorption geometry of free-base porphyrins on Cu(111)." Chemical Communications 53.58 (2017): 8207-8210.
- Schmitt, Tobias, et al. "Adsorption and Intermolecular Interaction of Cobalt Phthalocyanine on CoO(111) Ultrathin Films: An STM and DFT Study." Journal of Physical Chemistry C 121.5 (2017): 2889-2895.
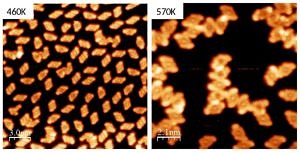
We have a strong interest in understanding the role a solid surface plays in chemical reactions. To address this topic related to catalysis we regard the single crystal surfaces as model catalysts that are well controlled on the atomic scale. Employing our surface science methods we study on the one hand the reactions that small test molecules (like CO, CO2, NO2,…) might undergo on (nanostructured) metal oxide surfaces. The insights from these experiments might become helpful for the design of catalysts that remove dangerous substances from the (exhaust) air or allow to produce climate-neutral fuels. On the other hand, we also investigate the possible covalent bonding reactions that occur for larger (organic) molecules forming supra-molecular nanostructures like ribbons or eventually two-dimensional structures with new functionalities.
Contact
- Prof. Dr. M. Alexander Schneider (Website: https://www.fkp.physik.nat.fau.eu/person/m-alexander-schneider/)
Publications
- Xiang, Feifei, Anja Gemeinhardt, and M. Alexander Schneider. "Competition between Dehydrogenative Organometallic Bonding and Covalent Coupling of an Unfunctionalized Porphyrin on Cu(111)." Acs Nano 12.2 (2018): 1203-1210.
The funCos project investigates porphyrins, which are essential for the binding and transport of oxygen in biological systems and also excellent electron acceptors in organic solar cells and donor-acceptor systems in general. To avoid a strong interaction of the molecules with the surface, which concealed their intrinsic properties in many “traditional” surface science experiments, we support the porphyrins by an oxide surface that they interact with very weakly.
A finished Emerging Fields Initiative project explored the ultrafast singlet fission process in bulk-like organic layers. In the singlet fission process, an optically excited electron-hole pair, called an exciton, transforms into two excitons which are protected from further energy losses. The process has the potential to enhance the efficiency of next-generation photovoltaics.
For the future, we plan to expand our research activities to extended molecules serving as models for Synthetic Carbon Allotropes.
Contact
Project funding
Functional Molecular Structures on Complex Oxide Surfaces (FOR 1878)
Publications